


On dilution, the final volume and concentration of the solution changes to V final and C final, respectively.
Consider the initial volume and concentration of the solution as V initial and C initial, respectively. On addition of the solvent, the quantity of the solution increases. In order to dilute a given solution, solvent is added to the original concentrated solution. The number of moles of solute remain the same before and after dilution. Molarity of a solution is higher when it is concentrated. Concentration is the inverse of dilution. The amount of solute dissolved in any solvent is termed as the concentration. Dilution reduces the ‘ph’ level of the chemical. It is diluted with water before consumption. This process is carried out in day-to-day life. Therefore, a solvent is added to such solutions to make them diluted. The concentration level of many solutions is much more than the desired level. Reducing the concentration of any chemical (solution, gas, vapor) is called dilution.
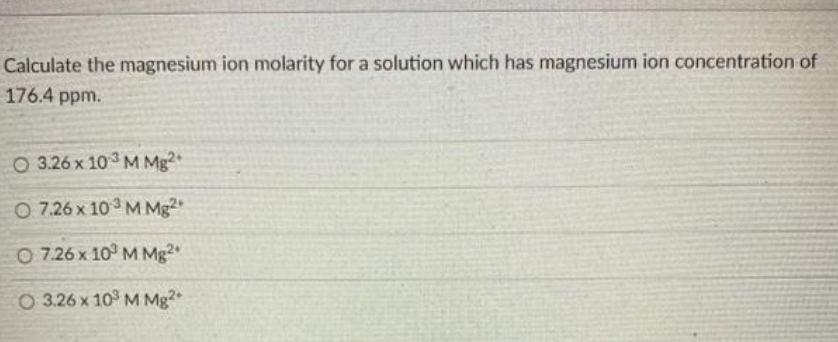
HOW TO CALCULATE PPM WITH MOLARITY HOW TO
In order to understand how to calculate the dilution factor from a given concentration value, we need to first understand a few terms. An example can be salt dissolved in water. The forces responsible for the binding of a solute and solvent to form a solution are ‘van der waals force’ or ‘hydrogen bonds’.Īccording to chemistry principles, a solute and solvent combine to form a solution. This ScienceStruck post shows how to calculate the dilution factor of solutions, given their initial and final concentrations. Hence, they are diluted using solvents in order to reduce their ‘ph’ value. Parts per million also can be expressed as milligrams per liter (mg/L).Chemicals in concentrated forms are seldom useful. Ppq is more a theoretical construct than a useful measurement, chances are you will never see it in use. Generally speaking it is very similar to weight percentage - 1% w/w means 1 gram of substance per every 100 g of sample and it is (although very rarely) named pph - parts per hundred. Parts Per Million(PPM) and Parts per Billion (PPB): "Parts per" is a convenient notation used for low and very low concentrations. The mole fraction of solute i is written as X i. Mole Fraction: The mole fraction of a single solute in a solution is simply the number of moles of that solute divided by the total moles of all the solutes/solvents. The weight percent is designated by Wt% and sometimes w/w%. This can be in grams or kilograms as long as the units of both are expressed in the same manner. Weight Percent(or Mass Percent): The weight percent of a solution is calculated by taking the mass of a single solute and dividing it by the total mass of the solution. Molality is designated by a lower case "m". Molality: The molality of a solution is calculated by taking the moles of solute and dividing by the kilograms of solvent. Molarity: The molarity of a solution is calculated by taking the moles of solute and dividing by the liters of solution. But don't become too reliant on it since it will not be available during exams. I have added a conversion calculator at the bottom of this page to help you check your homework etc. You should know the meaning of each of these terms and more importantly how to convert from one to the other. There are Five main ways we describe the concentration of solutions: 1) Molarity 2) Molality 3) Weight Percent 4) Mole Fraction and 5) Parts Per Million or Billion. The problems that might occur here stem from how much of this language you actually remember and whether or not you can apply what you remember to new problems. Fortunately, for discussing solutions, a great deal of the language was already covered in CHM1045. Whenever we begin to discuss a new subject we have to learn the language that accompanies it.


 0 kommentar(er)
0 kommentar(er)
